Converting cancer to a manageable, chronic condition
Learn MoreInspirna’s mission is to target previously untreatable common cancers with small molecules and biologics by exposing newly discovered drivers of metastasis. Through our proprietary and clinically-validated RNA-DRIVEr™ platform and microRNA mapping, Inspirna reveals new cancer drivers in areas of high unmet need.
RNA-DRIVEr™ = RNA Dysregulated Drug Target In Vivo Elucidation
News
Inspirna Announces Appointment of Karim Benhadji, M.D., as Chief Medical Officer and Retirement of Robert Wasserman, M.D.
NEW YORK – May 21st, 2024 – Inspirna, Inc., a clinical stage biopharmaceutical company developing first-in-class cancer therapeutics,… Read More
Inspirna to Present at the 42nd Annual J.P. Morgan Healthcare Conference
New York, NY – January 4, 2024 – Inspirna, Inc., a clinical stage biopharmaceutical company developing first-in-class cancer therapeutics,… Read More
Inspirna Partners with Merck KGaA, Darmstadt, Germany, to Accelerate Global Development of Ompenaclid (RGX-202)
Inspirna to receive $45 million in upfront payment for ex-US exclusive license and US co-development option to ompenaclid… Read More
RNA-DRIVEr™ Discovery Platform
Our proprietary and clinically-validated RNA-DRIVEr™ platform enables the discovery of previously unknown cancer drivers that can be targeted by small molecules and biologics.
Our aim is to provide more practical treatment options that prolong survival without decreasing patients’ quality of life. Inspirna is developing medicines with oral dosing and safety profiles that we believe will become seamless and simple additions to the existing standard-of-care.
Clinical and preclinical pipeline
Inspirna’s pipeline includes a first-in-class oral small molecule in Phase 2 development for patients with RAS mutant colorectal cancer (CRC), ompenaclid (RGX-202) as well as another first-in-class oral small molecule in Phase 1b/2 development for patients with non-small cell lung cancer (NSCLC) and endometrial cancer, abequolixron (RGX-104).
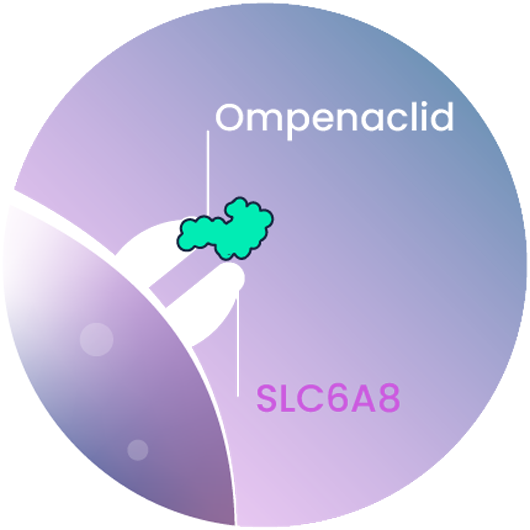
Ompenaclid (RGX-202)
Ompenaclid (RGX-202) is an oral small molecule inhibitor of SLC6A8 that induces apoptosis (cell death) of colorectal cancer cells.
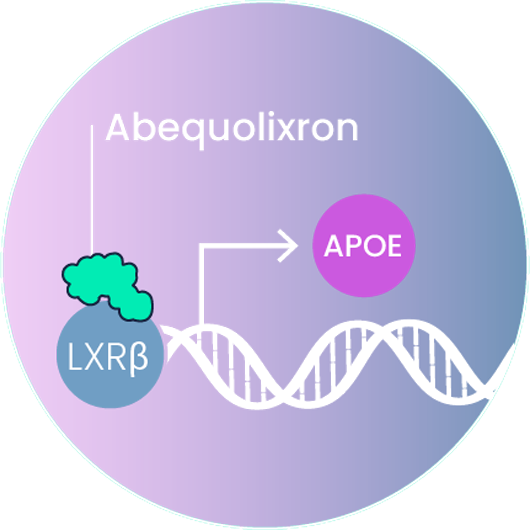
Abequolixron (RGX–104)
Abequolixron (RGX-104) is an oral small molecule activator of LXR/APOE that inhibits the growth of tumor blood vessels (angiogenesis) and inhibits tumor myeloid cells to enhance the immune response against tumors.
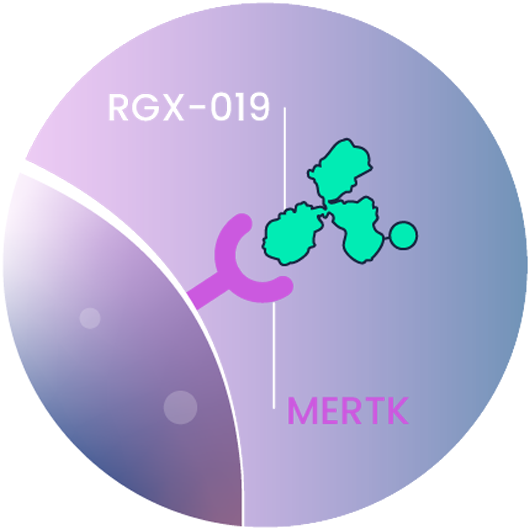
RGX-019
RGX-019-MMAE is a monoclonal antibody-drug conjugate (ADC) that targets MERTK to selectively kill cancer cells and targets tumor associated macrophages.


