
Inspirna has multiple therapies in development for the treatment of difficult to treat cancers.
These novel drug candidates are being developed in clinical settings of high unmet medical need, including RAS mutant colorectal cancer (CRC) as well as non-small cell lung cancer (NSCLC) and are being studied for the treatment of patients that harbor certain characteristics associated with clinical activity in completed Phase 1 studies.
Pipeline
Ompenaclid (RGX-202 Europe RCT*)
CKB/SLC6A8Ompenaclid (RGX-202 USA)
CKB/SLC6A8Abequolixron (RGX-104)
APOERGX-019 ADC
MERTKUndisclosed
Ompenaclid (RGX-202)
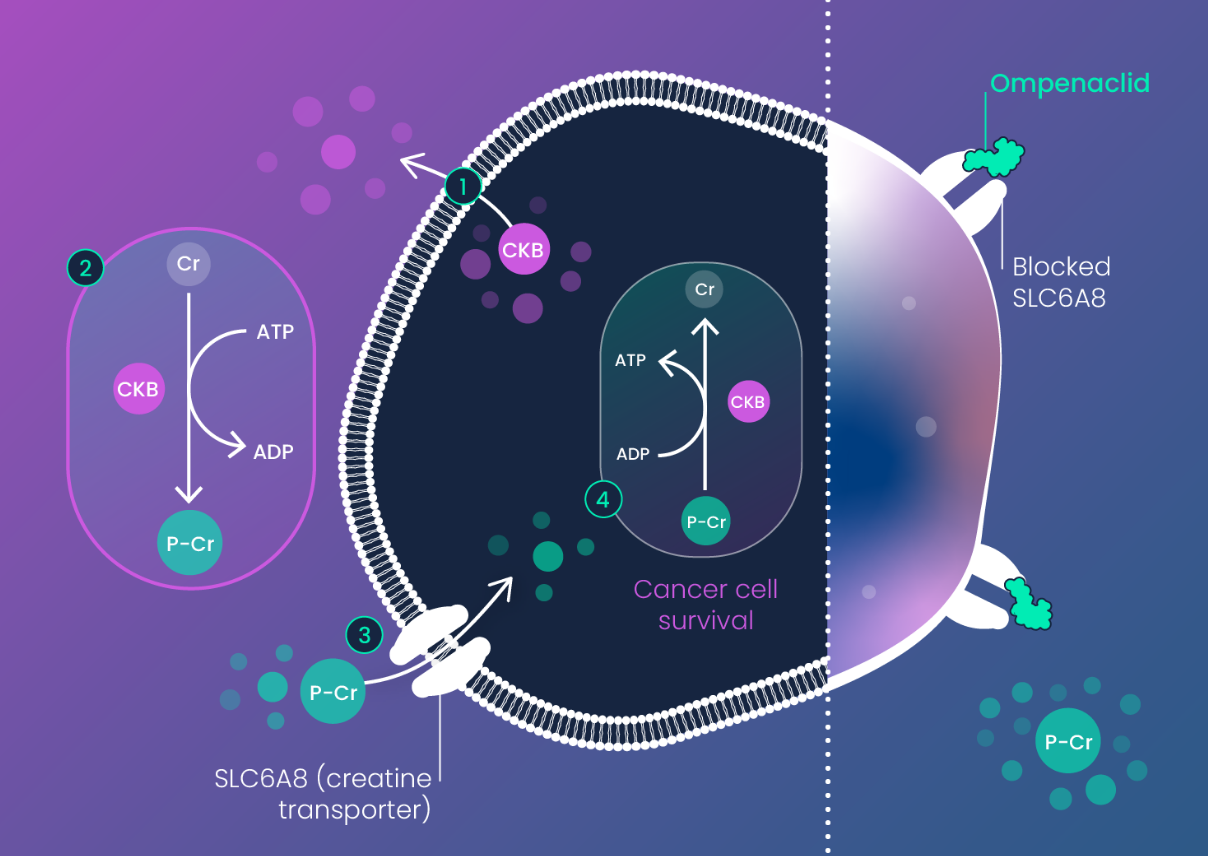
Creatine Kinase-B (CKB) was revealed as an RNA dysregulated cancer driver in RAS mutant colorectal cancer (CRC) by Inspirna’s RNA-DRIVEr™ platform. CKB is activated in response to tumor hypoxia (low oxygen) to enable cancer cells to generate ATP and other nucleotides via phospho-creatine.
Phospho-creatine is imported into CKB expressing (CKB+) cancer cells via the creatine transporter SLC6A8.
In the setting of tumor hypoxia (which is a common feature of CRC) ompenaclid (RGX-202)-mediated depletion of phospho-creatine (P-Cr) impairs synthesis of ATP and other nucleotides that are required for colorectal cancer cell survival, resulting in potent induction of apoptosis (cancer cell death).
- Cancer Cell Death
Since healthy (non-tumor) tissue is not hypoxic under normal conditions, inhibition of SLC6A8 by ompenaclid (RGX-202) largely leaves healthy tissue unaffected, providing a wide therapeutic window.
Ompenaclid (RGX-202) is currently being tested in combination with the standard-of-care regimen FOLFIRI and bevacizumab in a Phase 1b/2 clinical trial for the 2nd line treatment of patients with advanced colorectal cancer whose tumors have a mutation in the RAS gene (~40-45% of colorectal cancers).
Learn more about Inspirna’s collaboration with Merck KGaA, Darmstadt, Germany to develop ompenaclid in difficult to treat cancers.
Clinical Trials
A Phase 2 randomized control trial of ompenaclid (RGX-202) based in Europe, and an additional Phase 1b/2 clinical study, are in progress in patients with advanced colorectal cancer (CRC) whose tumors have a mutation in the RAS gene.
Abequolixron (RGX-104)
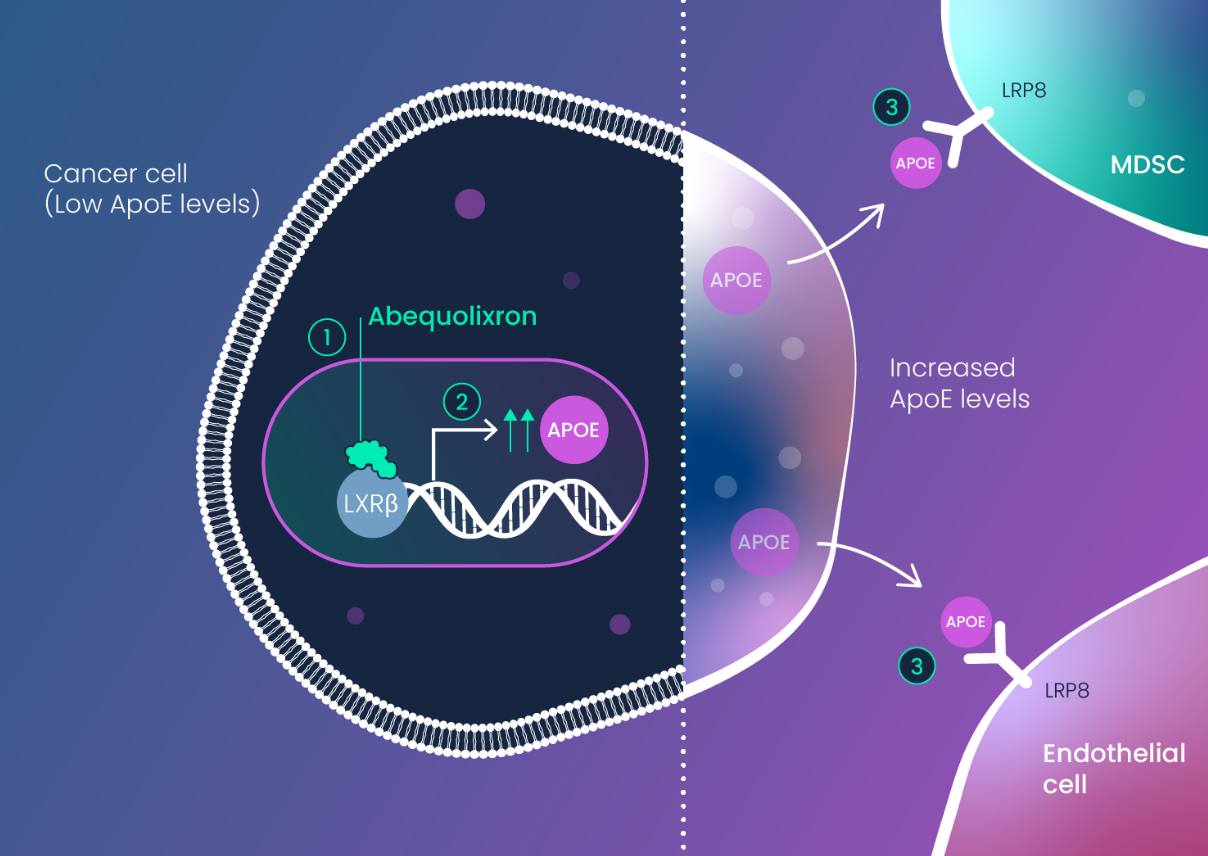
APOE was revealed by the miRNA-DRIVEr platform as a tumor suppressor that becomes silenced at the protein level by RNA dysregulation. APOE dysregulation is influenced by genetic variants of APOE (E2 and E4) which are present collectively in ~40% of the population.
APOE dysregulation results in two major effects: 1) increased tumor angiogenesis and 2) expansion of immune-suppressive myeloid cells (MDSCs, M2 TAMs, tolerogenic DCs).
- MDSC Depletion
- Dendritic Cell Activation
- T Cell Activation
- Angiogenesis Inhibition
This next-generation therapy has two key effects on the innate immune system that drive tumor immunity: it depletes myeloid-derived suppressor cells (MDSCs) and stimulates dendritic cells (DCs). MDSCs block the ability of T cells to become active, while stimulated DCs are required for proper activation (priming) of T cells.
By effecting these two types of innate immune cells (MDSCs and DCs), abequolixron (RGX-104) can unleash tumor-targeting T cells to induce anti-tumor activity.
Clinical Trials
Abequolixron is currently being tested in a Phase 1b/2 clinical trial for the treatment of patients with advanced non-small cell lung cancer (NSCLC) in combination with the standard-of-care therapy docetaxel. For more information about our clinical trial, visit clinicaltrials.gov.
RGX-019 ADC
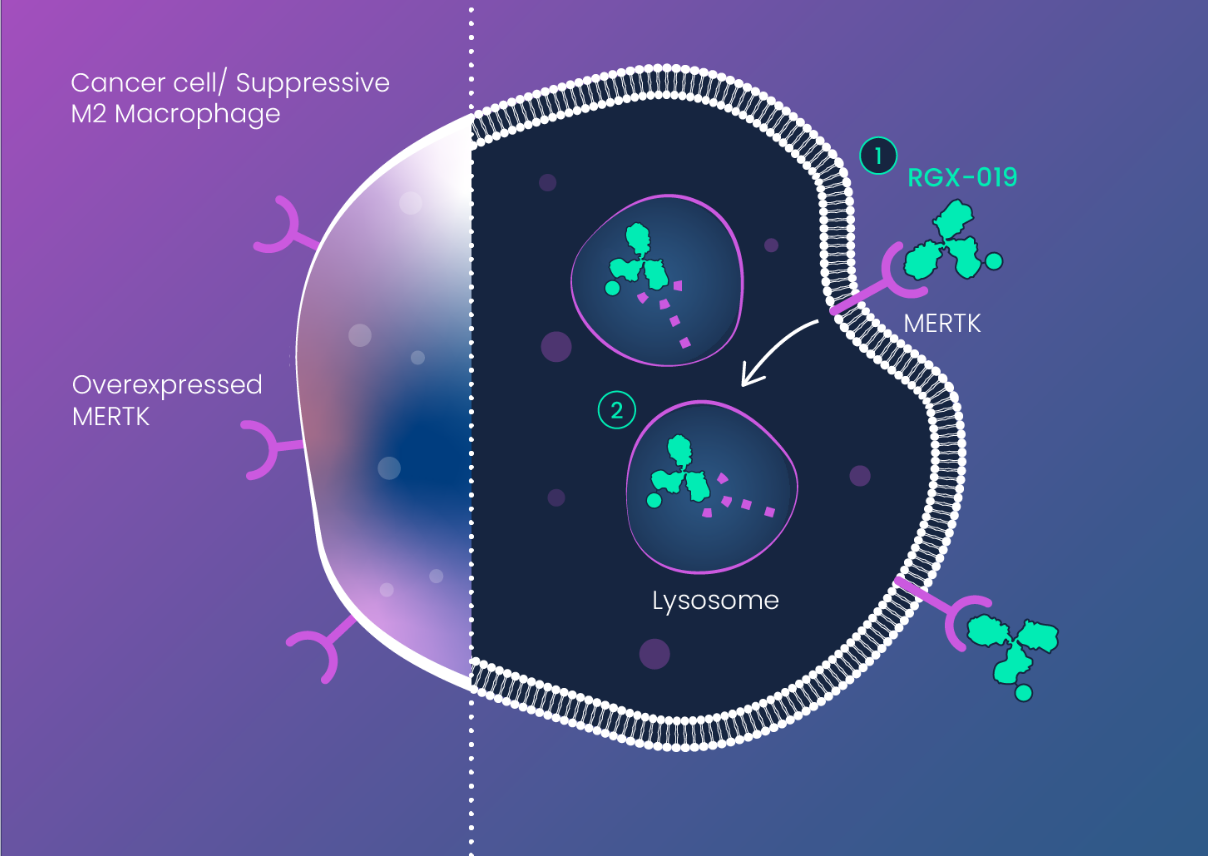
The miRNA-DRIVEr platform revealed that RNA dysregulation results in activation (increased expression) of MERTK in breast cancer (TNBC).
In addition to TNBC, MERTK is highly expressed in several other solid and liquid cancers, including NSCLC, melanoma, AML, and MM. MERTK activation causes direct effects on cancer cells (proliferation, migration) as well as immune suppressive effects through its action on tumor associated macrophages (TAMs).
MERTK is also required for maintenance of the retinal pigment epithelium (RPE) of the eye, hampering development of MERTK inhibitors which can result in retinal toxicity. RGX-019’s unique mechanism-of-action provides a wide therapeutic window that avoids retinal toxicity and enables an antibody-drug conjugate approach for effective targeting of MERTK expressing tumors.
- Reduced Cancer Cell Proliferation
- M1 Activation

